ঔষধের বিস্তারিত বা বিকল্প ঔষধ জানতে ঔষধের নাম দিয়ে সার্চ দিন। যেমন- Napa বা Alatrol বা Amodis
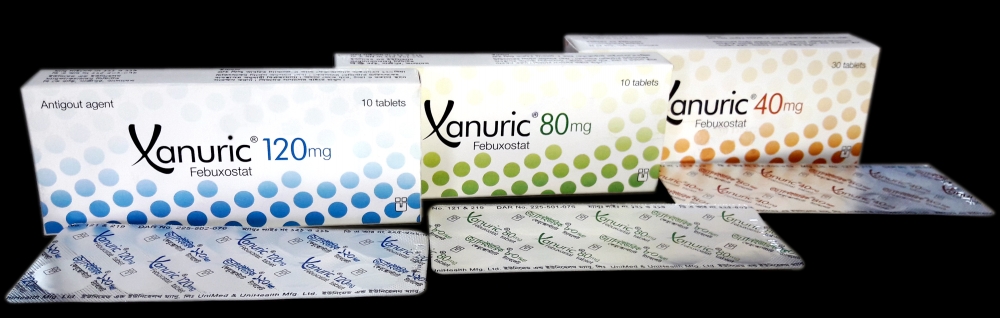
Xanuric 40mg
TabletFamotidine
Unimed Unihealth MFG. Ltd
Other Strength:
- Xanuric 80mg
- Xanuric 120mg
Alternative:
- Yamadin 40mg
- Famotack 40mg
Xanuric
Presentation
Xanuric 40mg tablet: Yellow, round shaped, film coated tablet; each tablet contains Febuxostat INN 40mg.
Xanuric 80mg tablet: Green, barrel shaped, film coated tablet; each tablet contains Febuxostat INN 80mg.
Xanuric 120mg tablet: Blue, oblong shaped, film coated tablet; each tablet contains Febuxostat INN 120mg.
Indication
Xanuric (Febuxostat) tablets are indicated for the chronic management of hyperuricemia in patients with gout. Xanuric (Febuxostat) tablets are not recommended for the treatment of asymptomatic hyperuricemia.
Dosage and administration
The recommended starting dose is one Xanuric 40 (Febuxostat 40mg) tablet once daily. For patients who do not achieve a serum uric acid less than 6 mg per dL after 2 weeks with Xanuric 40 (Febuxostat 40mg) tablet,one Xanuric 80 (Febuxostat 80 mg) tablet once daily is recommended. If serum uric acid less than 6 mg per dL after 2-4 weeks with Xanuric 80 (Febuxostat 80mg) tablet, then one Xanuric 120 (Febuxostat 120mg) tablet once daily is recommended.
Tumor Lysis Syndrome: The recommended dose is one Xanuric 120 (Febuxostat 120mg) tablet once daily.Xanuric 120 (Febuxostat 120mg) tablet should be started two days before the beginning of cytotoxic therapy and continued for a minimum of 7 days. However treatment may be prolonged up to 9 days according to chemotherapy duration as per clinical judgment.
Gout Flares: Gout flares may occur after initiation of Xanuric (Feboxostat) tablets due to changing serum uric acid levels resulting in mobilization of urate from tissue deposits. Flare prophylaxis with a non-steroidal anti-inflammatory drug (NSAID) or colchicine is recommended upon initiation of Xanuric (Febuxostat) tablets. If a gout flare occurs during treatment, Xanuric (Feboxostat) tablets need not be discontinued. The gout flare should be managed concurrently, as appropriate for the individual patient.
Renal impairment: No dose adjustment is necessary when administering Xanuric (Febuxostat) in patients with mild to moderate renal impairment.
Hepatic impairment: No dose adjustment is necessary in patients with mild to moderate hepatic impairment.
Method of administration: Oral use. Xanuric (Feboxostat) tablets can be taken with or without food.
Contra-indications, warnings, etc.
It is contraindicated for those patients have hypersensitivity to Febuxostat.
Precautions and warnings: Cardio-vascular disorders: Treatment with Febuxostat in patients with ischaemic heart disease or congestive heart failure is not recommended. Patient undergoing chemotherapy for hematologic malignancies at intermediate to high risk of Tumor Lysis Syndrome treated with Feboxostat should be under cardiac monitoring as clinically appropriate. Medicinal product allergy/ hypersensitivity: Severe hypersensitivity reactions, including Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) were associated with fever, haematological, renal or hepatic involvement in some cases. Patients should be advised of the signs and symptoms and monitored closely for symptoms of allergic/ hypersensitivity reactions. Febuxostat treatment should be immediately stopped if serious allergic/hypersensitivity reactions, including Stevens-Johnson Syndrome. If patient has developed allergic/ hypersensitivity reactions including Stevens-Johnson Syndrome and acute anaphylactic reaction/shock, Febuxostat must not be re-started in this patient at any time. Acute gouty attacks (gout flare): Febuxostat treatment should not be started until an acute attack of gout has completely subsided. Continuous treatment with Febuxostat decreases frequency and intensity of gout flares. Xanthine deposition: In patients in whom the rate of urate formation is greatly increased (e.g. malignant disease and its treatment, Lesch-Nyhan syndrome) the absolute concentration of xanthine in urine could, in rare cases, rise sufficiently to allow deposition in the urinary tract. As there has been no experience with Febuxostat, its use in patients with Lesch-Nyhan syndrome is not recommended.
Mercaptopurine/Azathioprine: Febuxostat use is not recommended in patients concomitantly treated with Mercaptopurine / Azathioprine. Where the combination cannot be avoided patients should be closely monitored. A reduction of dosage of Mercaptopurine or Azathioprine is recommended in order to avoid possible haematological effects. Theophylline: Febuxostat 40/80mg can be used in patients concomitantly treated with theophylline without risk of increasing theophylline plasma levels. No data is available for Febuxostat 120mg. Liver disorders: Liver function test is recommended prior to the initiation of therapy with Febuxostat and periodically thereafter based on clinical judgment. Thyroid disorders: Caution is required when Febuxostat is used in patients with alteration of thyroid function. Lactose: Febuxostat tablets contain lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Drug interactions:
Mercaptopurine/Azathioprine: On the basis of the mechanism of action of Febuxostat on XO inhibition concomitant use is not recommended. Inhibition of XO by Febuxostat may cause increased plasma concentrations of these drugs leading to toxicity. Rosiglitazone/CYP2C8 substrates: Co-administration of Febuxostat with rosiglitazone or other CYP2C8 substrates is not expected to require any dose adjustment for those compounds. Naproxen and other inhibitors of glucuronidation: Febuxostat metabolism depends on Uridine Glucuronosyl Transferase (UGT) enzymes. Medicinal products that inhibit glucuronidation, such as NSAIDs and probenecid, could in theory affect the elimination of Febuxostat. Febuxostat can be co-administered with naproxen with no dose adjustment of Febuxostat or naproxen being necessary. Inducers of glucuronidation: Potent inducers of UGT enzymes might possibly lead to increased metabolism and decreased efficacy of Febuxostat. Monitoring of serum uric acid is therefore recommended 1-2 weeks after start of treatment with a potent inducer of glucuronidation. Conversely, cessation of treatment of an inducer might lead to increased plasma levels of Febuxostat. Colchicine / Indometacin / Hydrochlorothiazide / Warfarin: Febuxostat can be co-administered with colchicine or indomethacin with no dose adjustment of Febuxostat. No dose adjustment is necessary for hydrochlorothiazide or warfarin when administered with Febuxostat . Antacids: Concomitant ingestion of an antacid containing magnesium hydroxide and aluminium hydroxide has been shown to delay absorption of Febuxostat (approximately 1 hour) and to cause a 32% decrease in Cmax, but no significant change in AUC was observed. Therefore, Febuxostat may be taken without regard to antacid use.
Use in pregnancy and lactation:
Pregnancy: Febuxostat should not be used during pregnancy. Breast-feeding: It is unknown whether Febuxostat is excreted in human breast milk. Febuxostat should not be used while breast-feeding. Fertility: The effect of Febuxostat on human fertility is unknown.
Effects on ability to drive and use machines: Somnolence, dizziness, paraesthesia and blurred vision have been reported with the use of Febuxostat. Patients should exercise caution before driving, using machinery or participating in dangerous activities until they are reasonably certain that Febuxostat does not adversely affect performance.
Side effects:
The most commonly reported adverse reactions are gout flares, liver function abnormalities, diarrhoea, nausea, headache, rash and oedema. These adverse reactions were mostly mild or moderate in severity. Rare serious hypersensitivity reactions to Febuxostat have observed.
Overdose:
Patients with an overdose should be managed by symptomatic and supportive care.
Pharmaceutical precautions
Store in a cool and dry place, protected from light
Packaging quantities
Xanuric 40mg tablet: Cartons containing 30 tablets in Alu-Alu blister pack
Xanuric 80mg tablet: Cartons containing 10 tablets in Alu-Alu blister pack
Xanuric 120mg tablet: Cartons containing 10 tablets in Alu-Alu blister pack
এই পাতাটি ৬৫ বার দেখা হয়েছে
রাজডক কী?
ফ্রী সদস্য হোন Click Here
ডাক্তার হিসাবে যোগদান করতে Click Here
নার্স / টেকনোলজিস্ট হিসাবে যোগদান করতে Click Here
ফ্রী সদস্য হোন Click Here
ডাক্তার হিসাবে যোগদান করতে Click Here
নার্স / টেকনোলজিস্ট হিসাবে যোগদান করতে Click Here

